Tetrahydropalmatine: The Next “Natural” Drug Slipping Into Vape Shops

What This Is — and Why It Matters Now
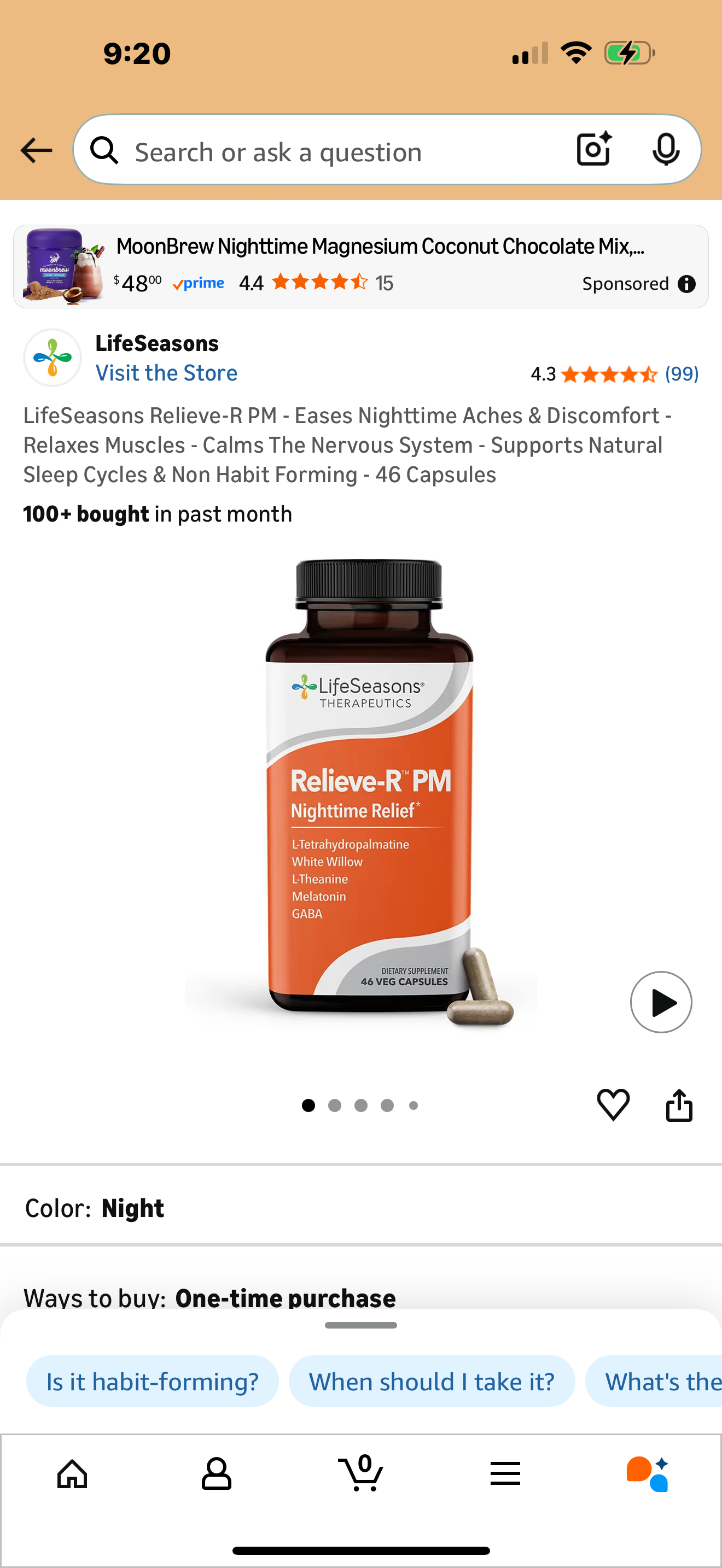
Tetrahydropalmatine—often listed as L-tetrahydropalmatine (L-THP) or disguised under the plant name Corydalis—is not a vitamin, nutrient, or benign herb. It is a psychoactive alkaloid that acts directly on the brain’s dopamine system. In research settings overseas, it has been studied as a sedative, analgesic, and experimental treatment for substance withdrawal. In the United States, however, it is not an FDA-approved drug—yet it is increasingly being sold in sleep aids, calming supplements, and vape-shop products with little to no warning about what it actually does.
Parents and lawmakers should understand this clearly: this compound behaves like a drug, but it is being sold as a supplement.
What People Are Using It For
Tetrahydropalmatine produces effects that many people actively seek, especially during times of stress, anxiety, and substance use cycling. Research shows it can:
- Sedate the brain and blunt stimulation
L-THP blocks dopamine D1, D2, and D3 receptors and alters other neurotransmitter systems, producing mental quiet, emotional flattening, and reduced reactivity. - Promote sleep without a prescription
Users often describe it as stronger than melatonin and comparable to prescription sedatives—an immediate red flag for an over-the-counter product. - Reduce anxiety and emotional intensity
Dopamine blockade can feel calming to people overwhelmed by stress, depression, or withdrawal from other substances. - Dull pain and bodily discomfort
Both animal and human studies document analgesic effects, including reduced neuropathic and withdrawal-associated pain. - Smooth withdrawal symptoms
Scientific literature describes its ability to reduce craving, hyperalgesia, and withdrawal severity from opioids and stimulants—effects that make it attractive to people cycling between substances.
These effects are not nutritional. They are pharmacologic.
Why This Is Showing Up in Vape Shops
Tetrahydropalmatine fits perfectly into the modern vape-shop business model.
First, it occupies the “legal intoxication” gap. It is psychoactive, non-scheduled, poorly understood by regulators, and easy to hide behind botanical labeling. That combination makes it attractive to retailers who already sell mood-altering products without prescriptions.
Second, it appeals to the same customer base. Vape shops routinely serve people looking for relief from anxiety, insomnia, pain, stress, or withdrawal—often young adults. A compound that quiets the brain and body while being marketed as “natural” sells easily.
Third, it can be falsely positioned as safer than prescription drugs. Retailers and manufacturers frame it as a natural alternative to benzodiazepines, opioids, alcohol, or antidepressants. That framing ignores the reality that L-THP acts on the same central nervous system pathways as regulated medications.
Finally, it flies under the radar. Most parents, educators, and legislators have never heard of tetrahydropalmatine. Corydalis sounds harmless. That ignorance is not accidental—it is the business strategy.
The Real Risks: Liver Injury, Neurologic Effects, and Unknown Long-Term Harm
Clinical case reports now document drug-induced liver injury (DILI) associated with Corydalis-containing supplements. In multiple cases, liver enzymes rose after use, improved after stopping the supplement, and rose again when the product was restarted—an established signal of causality in toxicology. Some patients developed severe hepatitis requiring medical intervention.
Beyond liver injury:
- Comprehensive pharmacology reviews warn of potential cardiac and neurologic toxicity
- Dopamine blockade carries risks of sedation, hypotension, movement disorders, and mood changes
- Long-term and high-dose toxicity data are explicitly lacking
- Human studies cited by manufacturers were short-term, tightly controlled, and never intended to justify daily consumer use
There is no required liver monitoring.
There is no standardized dose.
There is no post-market safety surveillance.
The FDA Reality: This Is Not an Approved Dietary Ingredient
Under U.S. law, ingredients introduced after 1994 must be submitted to the FDA as New Dietary Ingredients (NDIs) with evidence of safety before being sold.
There is no evidence that tetrahydropalmatine has:
- An accepted NDI notification
- FDA acknowledgment of safety
- Approval for any medical use in the United States
The FDA has never approved tetrahydropalmatine as a drug, and it has never approved Corydalis extracts as lawful dietary ingredients. The human trials frequently cited by industry were conducted under medical supervision for limited durations and explicitly do not support over-the-counter use.
Selling this compound as a supplement exploits a regulatory loophole—and leaves families unprotected.
Why This Looks Like the Next Kratom, Tianeptine, or Phenibut
Every major unregulated drug trend follows the same pattern:
- Introduced quietly as a supplement
- Marketed for stress, sleep, or pain
- Migrates into vape shops
- Harm appears after widespread use
- Families ask how it was ever legal
Tetrahydropalmatine is already well into this cycle. We now have documented liver injury, clear central nervous system activity, and a growing retail footprint—all without FDA approval or safety oversight.
What Parents and Policymakers Should Understand
This is not about banning herbs. It is about preventing drug-grade psychoactive compounds from being sold as supplements.
If tetrahydropalmatine were safe enough for unrestricted retail sale, it would not need to hide behind plant names, proprietary blends, or imported raw materials. It would have FDA review, dosage standards, and clear warnings.
It has none of those.
What it does have is dopamine blockade, sedation, liver injury reports, and increasing availability in vape shops. That combination warrants immediate attention—before this becomes the next preventable public health failure.
Take Action
- Document products in your community. Photograph labels listing “tetrahydropalmatine,” “L-THP,” or “Corydalis.”
- Report to state authorities. Use MAHA’s directory to find your health department and consumer protection contacts: State Take Action.
- Ask lawmakers one question: why is a dopamine-blocking psychoactive compound being sold like a vitamin?
References
- Engman S, Puello F, Khoury K, Shah D. Corydalis and drug-induced liver injury: a series of two cases. Am J Gastroenterol. 2023;118(suppl):S2441–S2442.
- Hassan HE, Kelly DL, Honick M, et al. Pharmacokinetics and safety assessment of L-tetrahydropalmatine in cocaine users: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 2017;57(2):151-160. doi:10.1002/jcph.789.
- Liu L, Liu M, Zhao W, Zhao YL, Wang Y. Levo-tetrahydropalmatine: a new potential medication for methamphetamine addiction and neurotoxicity. Exp Neurol. 2021;344:113809. doi:10.1016/j.expneurol.2021.113809.
- Oleinichenko D, Ahn S, Song R, Snutch TP, Phillips AG. Morphine withdrawal-induced hyperalgesia in models of acute and extended withdrawal is attenuated by l-tetrahydropalmatine. Int J Mol Sci. 2023;24(10):8872. doi:10.3390/ijms24108872.
- Du Q, Meng X, Wang S. A comprehensive review on the chemical properties, plant sources, pharmacological activities, pharmacokinetic and toxicological characteristics of tetrahydropalmatine. Front Pharmacol. 2022;13:890078. doi:10.3389/fphar.2022.890078.